Special Purpose Exit Company (SPEC)
NOTCH3-CADASIL Therapeutics
NOTCH3-CADASIL Therapeutics is a company striving to discover drugs that reverse the defects caused by NOTCH3 variants associated with Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy' (CADASIL) that result in cerebrovascular disease and dementia. Currently there is no cure for CADASIL and current treatments focus on managing patient symptoms. As a result, there is a large unmet need in CADASIL patients for drugs that reverse the underlying molecular defects and compensate for altered NOTCH3 function.
Variant Pathogenicity
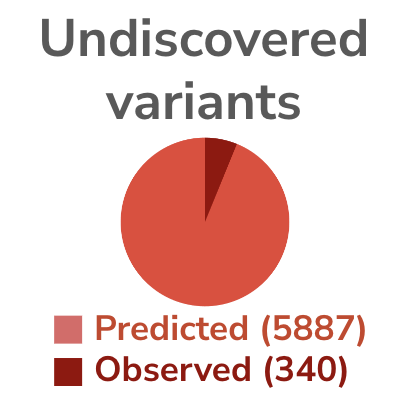
Of the 340 variants in NOTCH3 that are annotated as pathogenic, more than 45% are CADASIL associated. Yet of the 1079 total variants of NOTCH3 observed in ClinVar, more than 60% remain undecided for pathogenicity and are labeled as Variants of Uncertain Significance (VUS). With the average resolution of VUS leading to 30% being pathogenic, we can expect the numbers of pathogenic variants to grow by an additional 50% with resolution of VUS. However population predictions using AlphaMissese provides a pathogenicity heatmap suggesting another 15x more variants in NOTCH3 are pathogenic but have yet to be observed. Combined, there is significant room to grow a patient registry from improving diagnostic assessments.
Population Frequency
Quite a few pathogenic variants in NOTCH3 are seen at high frequency resulting in an estimated prevalence of 1 in 450 persons (33712516). As a result, there is a large population, possibly near 17 million, that could benefit from development of disease modifying therapeutics for CADASIL. However current patient registry databases have only a few thousand patients and therefore the affected population is significantly underdiagnosed. Currently the CureCADASIL Foundation has started curating a list of over 1000 patients, however a 10,000 patient population should be theoretically possible across the world.
Biomarkers
It is now well established that NOTCH3 variants causing CADASIL are frequently a disruption of the cysteine balance in the NOTCH3 extracellular domain (37602377). Specifically, genetic variations causing gain or loss of cysteine in one of the 34 the EGF repeat motifs in the extracellular domain of Notch3 are thought to disrupt the proper "handshake" with the Notch3 binding partners, the Jagged and Delta proteins, and the result is immature development of smooth muscle cells (34440858). Mechanistically, the disulfide imbalance leads to self-aggregation which possibly drives the formation of GOMs (Granular Osmiophilic Material) and disrupts synaptic signaling, however, aggregation as cause or consequence of the pathologic mechanism is still hotly debated (28293466). Regardless, thiol imbalance from loss or introduction of cysteines in NOTCH3 and its location along the extracellular domain is clearly correlated with CADASIL and its severity.
Loss of function experiments indicate NOTCH3 is required for vascular smooth muscle (VSM) differentiation (15545631). Furthermore, VSM maturation requires interaction with NOTCH3 receptor, the JAG1 gene ( 22204979, 34440858). Loss of function of NOTCH3 gene in orthologs of mice (15545631) and fish (23720232) cause vascular bleeding defects due to developmental abnormalities in capillary vesicle formation. One intriguing disease-mechanism hypothesis is the GOM formation in the smooth muscle layer destabilizes blood vessels and inhibits subsequent stem-cell mediated repair.
Drug Candidates
With CADASIL and its CNS phenotypes arising from vascular integrity issues, the ultimate drug screening platform will be to develop systems that examine the permeability of the blood-brain barrier. However, NOTCH signaling is so ancient and conserved, even models systems as simple as the C. elegans worm can potentially address the deficiencies caused by cysteine imbalance. We know the orthologs of NOTCH3 in worm, the glp-1 and lin-12 genes, each cause lethality when eliminated. What is not yet known is how well these KO animals can be rescued by gene replacement. We will be testing if the extracellular part of the worm gene (eg. lin-12) can be replaced with human coding sequence and still function. If so, we will then examine clinical variants for their detrimental effects on lin-12 signaling in the worm. Intriguingly, protein aggregation disorders can be easily modeled in C. elegans and often lead to early-onset locomotion defects (31951916). However, we will be also utilizing iPSC-derived tissue to create a CADASIL Blood-Brain Barrier (BBB) model. The complementarity of the C elegans model (fast) with the iPSC-based BBB model (accurate) is expected to yield modalities with high clinical translatability. Drug screens to be performed on models will include FDA-approved drug libraries and new entities like novel chemical entities, antisense oligonucleotides and conformation-specific antibodies.
NOTCH3 - CADASIL variants
List of NOTCH3 variants repeatedly seen in CADASIL:
NM_000435.3(NOTCH3):c.3296G>A (p.Cys1099Tyr)
NM_000435.3(NOTCH3):c.3226C>T (p.Arg1076Cys)
NM_000435.3(NOTCH3):c.3182G>A (p.Cys1061Tyr)
NM_000435.3(NOTCH3):c.3091C>T (p.Arg1031Cys)
NM_000435.3(NOTCH3):c.3062A>G (p.Tyr1021Cys)
NM_000435.3(NOTCH3):c.3043T>C (p.Cys1015Arg)
NM_000435.3(NOTCH3):c.3016C>T (p.Arg1006Cys)
NM_000435.3(NOTCH3):c.3011G>A (p.Cys1004Tyr)
NM_000435.3(NOTCH3):c.2989T>G (p.Cys997Gly)
NM_000435.3(NOTCH3):c.2953C>T (p.Arg985Cys)
NM_000435.3(NOTCH3):c.2951T>G (p.Phe984Cys)
NM_000435.3(NOTCH3):c.2129A>G (p.Tyr710Cys)
NM_000435.3(NOTCH3):c.1918C>T (p.Arg640Cys)
NM_000435.3(NOTCH3):c.1819C>T (p.Arg607Cys)
NM_000435.3(NOTCH3):c.1816T>C (p.Cys606Arg)
NM_000435.3(NOTCH3):c.1790G>A (p.Cys597Tyr)
NM_000435.3(NOTCH3):c.1774C>T (p.Arg592Cys)
NM_000435.3(NOTCH3):c.1759C>T (p.Arg587Cys)
NM_000435.3(NOTCH3):c.1732C>T (p.Arg578Cys)
NM_000435.3(NOTCH3):c.1672C>T (p.Arg558Cys)
NM_000435.3(NOTCH3):c.1630C>T (p.Arg544Cys)
NM_000435.3(NOTCH3):c.1594C>T (p.Arg532Cys)
NM_000435.3(NOTCH3):c.1547G>A (p.Cys516Tyr)
NM_000435.3(NOTCH3):c.1531T>A (p.Cys511Ser)
NM_000435.3(NOTCH3):c.1363T>C (p.Cys455Arg)
NM_000435.3(NOTCH3):c.1345C>T (p.Arg449Cys)
NM_000435.3(NOTCH3):c.1282T>C (p.Cys428Arg)
NM_000435.3(NOTCH3):c.1279C>T (p.Arg427Cys)
NM_000435.3(NOTCH3):c.1258G>T (p.Gly420Cys)
NM_000435.3(NOTCH3):c.1187C>G (p.Ser396Cys)
NM_000435.3(NOTCH3):c.1013G>T (p.Cys338Phe)
NM_000435.3(NOTCH3):c.994C>T (p.Arg332Cys)
NM_000435.3(NOTCH3):c.932G>T (p.Cys311Phe)
NM_000435.3(NOTCH3):c.779G>T (p.Cys260Phe)
NM_000435.3(NOTCH3):c.773A>G (p.Tyr258Cys)
NM_000435.3(NOTCH3):c.751T>C (p.Cys251Arg)
NM_000435.3(NOTCH3):c.665G>A (p.Cys222Tyr)
NM_000435.3(NOTCH3):c.617G>A (p.Cys206Tyr)
NM_000435.3(NOTCH3):c.602G>C (p.Cys201Ser)
NM_000435.3(NOTCH3):c.602G>A (p.Cys201Tyr)
NM_000435.3(NOTCH3):c.581G>A (p.Cys194Tyr)
NM_000435.3(NOTCH3):c.581G>T (p.Cys194Phe)
NM_000435.3(NOTCH3):c.580T>C (p.Cys194Arg)
NM_000435.3(NOTCH3):c.580T>A (p.Cys194Ser)
NM_000435.3(NOTCH3):c.554G>A (p.Cys185Tyr)
NM_000435.3(NOTCH3):c.553T>C (p.Cys185Arg)
NM_000435.3(NOTCH3):c.548G>T (p.Cys183Phe)
NM_000435.3(NOTCH3):c.547T>C (p.Cys183Arg)
NM_000435.3(NOTCH3):c.544C>T (p.Arg182Cys)
NM_000435.3(NOTCH3):c.521G>A (p.Cys174Tyr)
NM_000435.3(NOTCH3):c.520T>C (p.Cys174Arg)
NM_000435.3(NOTCH3):c.505C>T (p.Arg169Cys)
NM_000435.3(NOTCH3):c.457C>T (p.Arg153Cys)
NM_000435.3(NOTCH3):c.437G>A (p.Cys146Tyr)
NM_000435.3(NOTCH3):c.436T>C (p.Cys146Arg)
NM_000435.3(NOTCH3):c.431G>T (p.Cys144Phe)
NM_000435.3(NOTCH3):c.421C>T (p.Arg141Cys)
NM_000435.3(NOTCH3):c.397C>T (p.Arg133Cys)
NM_000435.3(NOTCH3):c.350G>T (p.Cys117Phe)
NM_000435.3(NOTCH3):c.350G>A (p.Cys117Tyr)
NM_000435.3(NOTCH3):c.323G>T (p.Cys108Phe)
NM_000435.3(NOTCH3):c.268C>T (p.Arg90Cys)
NM_000435.3(NOTCH3):c.245G>T (p.Cys82Phe)
NM_000435.3(NOTCH3):c.244T>C (p.Cys82Arg)
NM_000435.3(NOTCH3):c.224G>C (p.Arg75Pro)
NM_000435.3(NOTCH3):c.200G>T (p.Cys67Phe)
NM_000435.3(NOTCH3):c.194G>A (p.Cys65Tyr)
NM_000435.3(NOTCH3):c.194G>C (p.Cys65Ser)
NM_000435.3(NOTCH3):c.164G>A (p.Cys55Tyr)
NM_000435.3(NOTCH3):c.160C>T (p.Arg54Cys)
Special Purpose Exit Company (SPEC)
Devinebio creates entities (SPECs) to develop drug assets for a specific indications. Five areas are monitored per each SPEC: population, biomarkers, leads, IP, and regulatory. A SPEC’s assets are moved through the milestones of preIND, IND, preNDA and NDA. As a drug asset progresses, it becomes de-risked and it increases in value. Once a drug asset has achieved IND-enabled status, it will have matured enough to be ready for partnering with Pharma.